Regulatory reporting:
Improving processes while ensuring data integrity
Introduction
Regulatory reporting is an essential yet complex part of any hospital’s clinical data management process. By collecting and submitting data on core measures to regulatory bodies such as CMS and the Joint Commission, hospitals provide valuable information on health care quality. This in turn impacts hospitals’ reimbursements and accreditation and fosters improvements in patient care.
Despite its many benefits, regulatory reporting is fraught with challenges. Hospitals must meet compliance standards for multiple state and federal regulatory agencies, keep up with constantly changing guidelines, keep track of various deadlines for submitting data, and review and prepare data for submission. The time staff spends on these tasks leaves little room for other duties. From evaluating clinical quality data activities at more than 200 hospitals, Q-Centrix found that 58 percent of the staff time allocated for core measures was spent on data curation alone, leaving far less time for performance improvement, clinical care, and other activities.
These challenges underscore the need for hospitals to find solutions for their regulatory reporting efforts. This white paper discusses common regulatory reporting challenges, shares the experiences of a hospital partner that has implemented effective regulatory reporting processes while using Q-Centrix’s regulatory reporting enterprise platform, and offers suggestions on how hospitals can meet regulatory guidelines while prioritizing data integrity.
Barriers to effective regulatory reporting
Different standards and deadlines from multiple regulators
Between CMS, the Joint Commission, and other state and federal healthcare regulatory agencies, hospitals must track and adhere to multiple regulators’ specific standards, which can be challenging and time consuming. Keeping track of each regulator’s deadlines adds even more difficulty to the process.
Redundant processes
Regulatory bodies typically have varied requirements for how data should be reported or formatted, requiring staff to spend valuable time formatting the same data in different ways to meet these specific requirements. This duplicative work can make reporting more costly and complicated.
Changing guidelines
Just as the healthcare landscape is always evolving, so too are its many regulations. With every rule change, hospitals must race to keep up with the new standards, adjust their policies accordingly, and educate staff on new and revised rules.
Costly penalties
Mistakes in regulatory reporting add up quickly, as even minor reporting errors could potentially result in penalties or reduced incentives. If hospital staff are not well versed in reporting minutiae — or if the clinical data they use for regulatory reporting are inaccurate — hospitals may see expensive consequences.
Regulatory reporting at Lehigh Valley Health Network
Managing regulatory reporting for an enterprise health system can be particularly complex. Lehigh Valley Health Network (LVHN) is a 13-hospital health system based in eastern Pennsylvania. Cynthia Ciocco, RHIT, clinical outcomes manager at LVHN, uses Q-Centrix’s regulatory reporting platform to manage chart abstracted measures for both inpatient and outpatient quality reporting programs as well as Hospital-Based Inpatient Psychiatric Service (HBIPS). Ciocco and her team follow several practices to ensure a smooth and effective regulatory reporting process:
Weekly data imports: While chart-abstracted measure population was initially uploaded monthly, Ciocco and her team began importing data weekly from their electronic health record (EHR) to Q-Centrix. This allowed Ciocco to review the abstracted data in a more timely manner, review for any missed exclusion codes, identify areas for improvement, and regularly share reports with hospital leadership.
Customizing fields: Ciocco added custom fields for certain key data points in the regulatory reporting tool. During chart abstraction, her
abstractors enter additional clinical details in the User Defined Field section. Ciocco noted that these fields can be reported and help clinical teams better understand measure requirements and strategize improved care processes or documentation workflows, particularly for sepsis.
Process improvements: While LVHN’s regulatory reporting process is highly effective, Ciocco is always looking for ways to improve. Ciocco participates in focus groups with clinical teams involved in measure documentation to consider new ways to improve LVHN’s compliance efforts. Ciocco also partnered with other departments to educate staff on measure requirements. “I work very closely with the quality department, and working in tandem with them is very helpful,” said Ciocco. “They developed a sepsis PowerPoint learning for providers. I also worked with the HBIPS leadership team to develop a PowerPoint for each of those measures as well.”
Regulatory reporting with Q-Centrix
Q-Centrix’s regulatory reporting platform helps hospitals tackle regulatory compliance with ease. The reporting tool can be used for a number of CMS and the Joint Commission core measures.
For each measure, the tool provides users with administrative, analytical, reporting, and data collection tools to help hospitals submit data on time and identify issues in data collection, documentation, or care processes. Q-Centrix also helps hospital partners track submission deadlines and offers summaries and quarterly webinars detailing new and revised regulations.
Q-Centrix keeps its regulatory reporting tool up to date with changing guidelines and continually looks for new ways to make regulatory reporting easier for hospital partners.
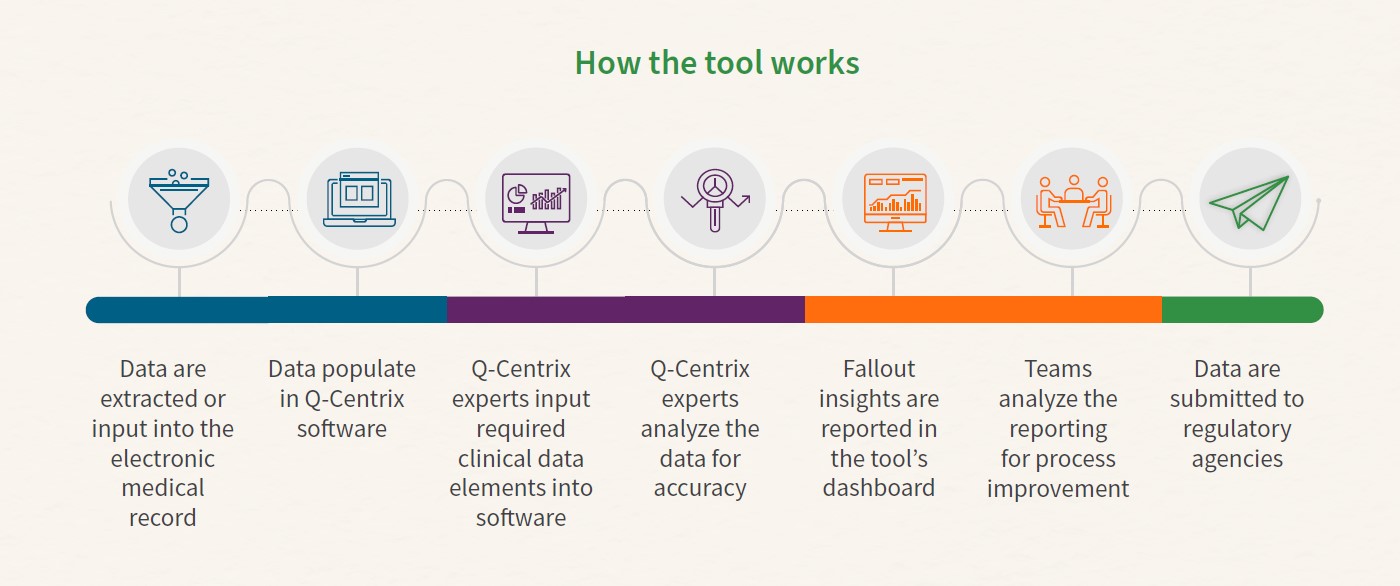
Best practices for regulatory reporting
Use compliance tools.
Relying on data collection and reporting tools to facilitate key regulatory compliance aspects can free up time for clinical staff to spend with patients. For example, Q-Centrix’s regulatory reporting platform offers accurate, on-demand reporting to help hospitals submit required data on time. Q-Centrix’s hospital partners are unanimous in finding the platform useful: a 2022 survey of hospital partners found that all 100 percent of respondents felt Q-Centrix’s regulatory reporting service was valuable to them and their team.
Partner with an expert.
Third parties can help hospitals navigate changing guidelines, multiple deadlines, and other nuances of submitting clinical data to various regulatory bodies. Cynthia Ciocco at LVHN mentioned that the final rule summaries and submission deadline calendars Q-Centrix provides helped her staff meet regulatory deadlines and stay up to date on new and revised standards.

Get the message out.
All staff involved in regulatory reporting should be clear on guidelines and expectations for proper compliance. At an enterprise health system such as LVHN, this may involve partnering with stakeholders across departments to help spread the message. For instance, Ciocco collaborated with other departments to develop PowerPoint slides educating staff on compliance practices. “The challenge is to get that education out to all the right people,” she explained.
Ciocco also worked with a physician who could champion the importance of compliance to other physicians throughout LVHN. “The best thing to do is to find that physician champion who will work to get the word out,” Ciocco said. Other ways to raise awareness about compliance may include posters, microsites, regular email communications, videos, and staff meetings.
Look for ways to improve existing processes.
With ever-changing guidelines, compliance is an ongoing process. Hospital leaders should stay on the lookout for new ways to improve their regulatory reporting practices. Ciocco shares detailed quality measure reports and participates in focus groups with clinical teams to discuss how LVHN could further its compliance efforts in HBIPS and sepsis. The HBIPS focus group included staff involved in documentation for HBIPS measures, such as department directors, case managers, social workers, PAs, and nursing leadership. Meanwhile, the sepsis focus group included emergency department leadership, physicians, PAs, nurses, and quality department colleagues.
Centralize clinical data practices.
Centralizing (streamlining practices and resource management in a facility or system to standardize operations) can assist with compliance, save on costs, and improve data integrity. When LVHN centralized its abstraction practices, the health system saw many benefits, including the ability to create uniform processes and standards.
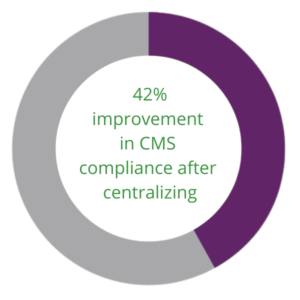
“Centralizing the abstraction process ensures the quality of guideline compliance across the network,” Ciocco explained. Similarly, when another Q-Centrix partner, a nonprofit health system in California, centralized its quality efforts, the health system saw its compliance for CMS improve by up to 42 percent in two years.
“Centralizing the abstraction process ensures the quality of guideline compliance across the network.”
- Cynthia Ciocco, RHIT, clinical outcomes manager at LVHN
Conclusion
Keeping up with changing regulations will continue to be a challenge. However, with the help of compliance tools and third-party experts—and by working with hospital staff to raise awareness about compliance and identify process improvements—hospital quality leaders can more easily adapt to rule changes and ease the burden on clinical and quality departments.