Increasing sepsis compliance through real-time exception reporting
Introduction
At least 1.7 million adults in the U.S. develop sepsis in a typical year, according to the Centers for Disease Control and Prevention. Given the life-threatening nature of sepsis, timely recognition and treatment are vital. Complying with sepsis core measures is crucial for improving patient outcomes, and reporting on compliance plays a key role in hospital reimbursements.
The health system that Q-Centrix partnered with, which is based in Texas and has more than 600 patient care centers, takes sepsis very seriously. The health system strives to deliver highquality patient care and adhere to sepsis core measures to obtain the best possible outcomes for its patients.
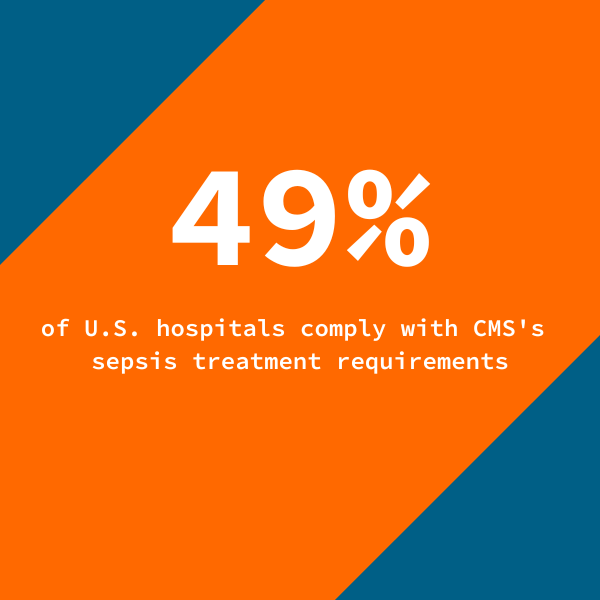
However, sepsis compliance rates suffered due to challenges in the reporting process. While physicians were taking the steps necessary to prevent and address sepsis, inconsistencies in documentation practices resulted in some situations being incorrectly categorized as fallouts. This is a barrier for many hospitals. CMS data show that just 49 percent of U.S. hospitals comply with CMS’s sepsis treatment requirements, with documentation issues cited as a common challenge.
Data integrity posed another challenge in the health system’s sepsis compliance efforts. Without a proven way to verify the quality of their clinical data, physicians were not sure whether they could trust the data they relied on to identify and monitor sepsis cases. To make significant improvements in sepsis compliance, the health system would need to look beyond standard compliance tools and seek out more advanced analytics technology with the ability to identify fallouts and exceptions, report on quality assurance, and analyze key trends.
To improve sepsis reporting outcomes and documentation, reduce fallouts, and ensure greater data integrity, the health system leveraged Q-Centrix’s clinical data expertise and analytics technology to implement improvements in their sepsis reporting process at one of their hospitals, a 431-bed acute care and trauma center. Hospital leaders hoped that by improving sepsis outcomes in this location, they could convince executives to implement this approach in all sites throughout the health system.
Goals
- Improve outcomes in sepsis measure reporting
- Implement advanced technology for identifying fallouts and exceptions
- Reduce fallouts and achieve higher CMS compliance for sepsis core measures
- Build the business case to implement a centralized sepsis approach across the health system
Challenges
Lack of trust in clinical data
Without a process to ensure data integrity, physicians were unsure whether the data were accurate
Difficulty identifying how and when fallouts occurred
The compliance tools available to the hospital did not identify fallouts or exceptions, making it difficult for the hospital to find ways to improve in these areas
Inconsistent documentation practices
Physicians did not always document crucial information in the correct place, leading to some situations being falsely categorized as fallouts simply because the relevant information was not in its proper place
Solutions
- Worked with Q-Centrix’s clinical data experts and used Q-Centrix’s market-leading data integrity analytics technology, including the Q-Card®
- In addition to reporting monthly quality assurance activities, the Q-Card® features a dashboard with key performance indicators, including historical trends in case volumes and fallouts
- This helped instill confidence in the quality of the hospital’s clinical data
- Identified fallouts in real-time using Q-Centrix’s Exception Reporting Tool
- This technology offers real-time, in-depth reporting beyond what typical registry participation provides, allowing hospitals to attribute fallouts to specific units or clinicians
- This enabled the hospital to quickly address and reduce fallouts and compliance concerns as they occurred
Outcomes
After working with Q-Centrix for six months, the hospital saw:
- Significant reduction in fallouts for several sepsis measures, including:
- Broad Spectrum or other Antibiotic
- Repeat Lactate
- Blood Cultures prior to Antibiotics
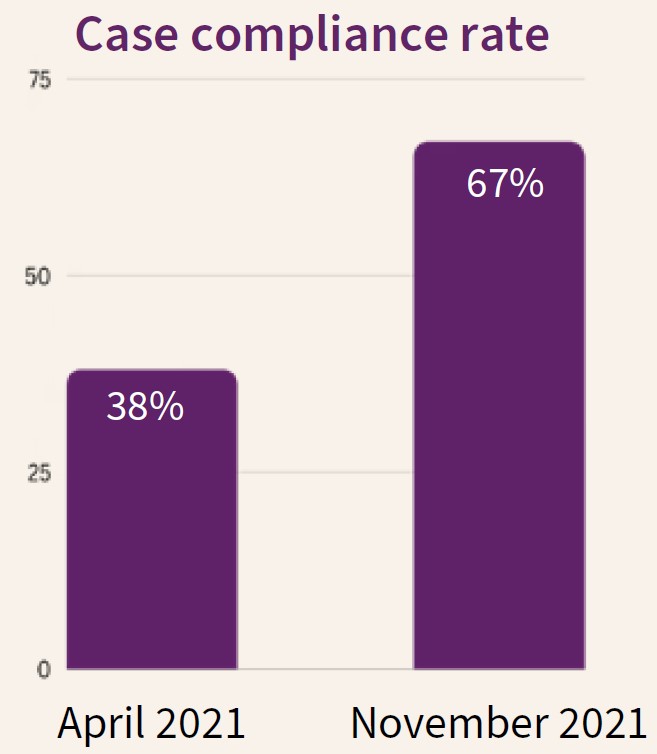
- Case compliance rates increased by 76 percent
- Greater physician confidence in the quality of the clinical data
- Improved documentation practices due to the Exception Reporting Tool’s real-time reporting technology
- Physicians received immediate feedback if their documentation practices resulted in a fallout, enabling them to adjust their approach to document exceptions correctly
Conclusion
The hospital’s sepsis process improvement initiative resulted in significantly fewer fallouts, higher compliance rates, and improved documentation practices. Using Q-Centrix’s clinical data expertise and data analytics technology ensured greater trust in the integrity of their clinical data. The Q-Card® reported on quality assurance activities monthly, and the Exception Reporting Tool helped alert physicians to documentation errors in real-time. Through these technologies, the hospital could access and develop custom reports, analyze trends, export executive summaries, and use these insights to improve processes.
The health system’s success in implementing this initiative at one hospital suggests that centralizing this approach throughout the health system could see even greater improvements and potentially position the health system as a leader in sepsis compliance.
With half of U.S. hospitals failing to comply with CMS’s sepsis treatment requirements, the health system’s approach to improve its sepsis reporting offers key lessons for hospital leaders looking to advance their sepsis efforts. By engaging with a third party to ensure data integrity, identify fallouts, and improve documentation practices, health systems can achieve higher compliance rates and uphold their commitment to patient safety.